Why researchers should use human embryo models with caution
[ad_1]
The first few weeks of a human embryo’s development are shrouded in mystery. Researchers cannot observe embryos that are nestled away in their mother’s womb until they are large enough to be detected by ultrasound, about three weeks after conception. Few human embryos are donated to research, and regulations in many countries prevent those few from being studied beyond a stage equivalent to 14 days of natural development.
Researchers need creative ways to study human development without using actual human embryos. One answer lies in stem cells, which, under the right conditions, can be coaxed to form embryo-like structures that can be studied in vitro.
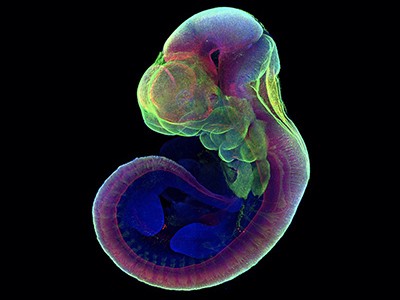
Now three teams writing in Nature1–3, along with others4–6, have used stem cells to generate models of the human embryo that are more advanced than those previously available. These new structures are what are known as integrated embryo models, because they contain tissues that are representative of parts of the embryo itself as well as the surrounding ‘extraembryonic’ structures such as the yolk sac that grow with the embryo and support its development. These models mimic a key week in human development, from just before the embryo implants into the uterus wall at about day 7, until up to or just past the 14-day mark, when the embryo starts preparing to form the first differentiated layers of tissue. This is a few days later than has been achieved by other integrated embryo models, and some of the latest models contain more tissue types than in previous attempts.
The next frontier for human embryo research
These structures add to a growing toolbox of embryo models7,8 that could help to uncover the events that lead to early pregnancy loss — a crucial area of study, given that about 60% of human pregnancies fail in the first 14 days, according to one estimate9. The hope is that they could help researchers to design better reproductive technologies, find ways to reduce miscarriages and treat congenital diseases.
But the current work also raises the controversial notion of a possible future in which embryo models could be grown for longer to produce something that would be considered ‘human’10,11. Although this is far from current reality, many researchers are concerned that media hype around such ideas might mislead the public into thinking that scientists are trying to grow humans from stem cells, and so erode their trust in scientific research.
To minimize the risk of future controversy, and avoid some of the practical and ethical challenges of generating integrated models, we argue that this approach should be used with caution. We call on researchers to carefully define the scientific questions they wish to address and to consider the most appropriate embryo model for their purpose. In many cases, less-controversial ‘non-integrated’ human embryo models that mimic only certain aspects of development can address pressing research questions equally well.
Integrated human embryo models
Human embryo models are considered to be ‘integrated’ if — like those recently reported — they contain embryonic and extraembryonic tissues that together give them the potential to produce something similar to an intact human embryo. Models are considered ‘non-integrated’ if they lack some of the tissue types needed for this. Integrated models fall into two categories on the basis of the developmental stages they aim to copy.
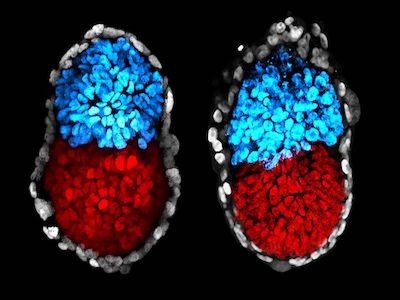
The first mimics the initial few days of human development, when the fertilized egg divides several times to produce a ball of ‘epiblast’ cells, which go on to form the embryo, and a surrounding sphere of extraembryonic cells that go on to generate the placenta. These models, called blastoids, are generated by confining human stem cells in microwells and treating them with chemicals to trigger their growth and differentiation. Blastoids are similar to equivalent-stage human embryos in their size, shape and gene-expression profile. Because blastoids can be generated in large numbers, they can be used to screen for chemicals that might improve in vitro fertilization (IVF) treatments12.

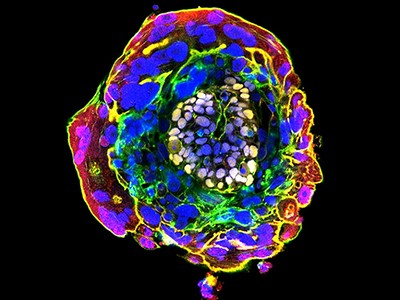
Human embryo models grown from stem cells look similar in shape and structure to natural embryos 14 days after conception.Credit: B. Oldak et al./Nature
One limitation of human blastoids is that, as with natural human embryos grown in culture, they currently do not develop well beyond a stage equivalent to when the natural embryo implants in the uterus wall. Yet the two to three weeks that follow implantation are particularly important. During this time, the embryo undergoes a process called gastrulation, in which the epiblast cells become organized into layers of embryonic tissues that define the basic body plan. The primitive heart, early brain and spinal cord, blood and placenta are then formed.
The second type of integrated human embryo model — which includes many of the models reported in the latest studies1–5 — is designed to mimic the phases of development after implantation, when the embryo begins to prepare for gastrulation. By skipping the first week of human development, these models bypass the implantation barrier faced by blastoids and develop to a stage equivalent to 14 days of natural embryo development. Some models are designed to mimic the entire human embryo1,3 including all the cell types needed to form the supporting membranes, which are crucial for a successful pregnancy (the amnion, yolk sac and placenta). Other approaches are less complete, because they exclude the ‘trophoblast’ cells required for placenta formation2,4,5.
These ‘post-implantation’ models are the only way to fully visualize the developmental events that are usually hidden in the uterus. But there are challenges associated with developing and using these approaches.
What is an embryo? Scientists say definition needs to change
There is no good standard against which to benchmark post-implantation models, because researchers cannot study natural human embryos — or even monkey embryos, which are the closest equivalent — in any depth after they implant in the womb. This makes it difficult to gauge how closely each model resembles normal human development.
Protocols are currently inefficient. Researchers at different laboratories start out with different kinds of stem cell and they culture the cells under different conditions, making it hard to compare how well each model replicates human development. Furthermore, integrated models aimed at mimicking post-implantation development often contain disorganized cell types and tissue structures1–5.
One reason that is commonly given for developing integrated post-implantation models is to study developmental events that occur in the weeks after gastrulation, such as the formation of the heart, the development of the placenta and the generation of the ‘neural tube’ tissue that goes on to form the brain and spine. This would require growing these models for a week or two longer in culture than is currently possible. Mouse embryo models grown in specialized culture chambers can grow past gastrulation and begin forming organ rudiments13,14 — lessons from these systems might be applicable to human models.
However, the fact that it might one day be possible to grow integrated human embryo models for extended periods of time raises serious ethical and regulatory concerns. Should these models be viewed and regulated as though they were human embryos? And, if so, should the 14-day restriction be applied?
Consider alternatives
Clearly, a high bar should be set for developing stem-cell-based models that are intended to replicate a full embryo. Researchers should ask themselves: ‘Am I making this model for strong scientific reasons?’, ‘Is there no other alternative system?’ and ‘Am I prepared to defend my work in the court of public opinion?’.
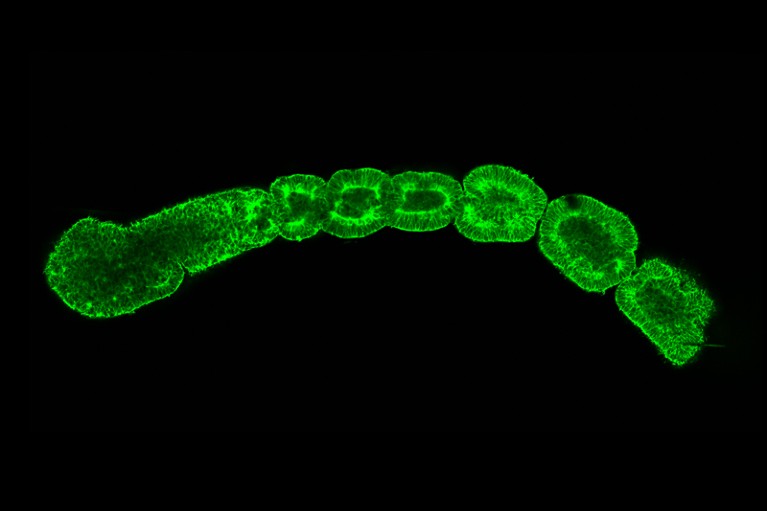
Human stem cells can be used to model individual aspects of development — for instance mimicking blocks of somite tissue from which vertebrae form.Credit: M. Sanaki-Matsumiya et al./Nature Commun. (CC BY 4.0)
Alternative stem-cell-based models are available that mimic some aspects of human development, including gastrulation and early organ formation7,8. These non-integrated models pose fewer ethical challenges than do integrated ones. They do not contain all the cell types needed for a human fetus to grow, but have several other useful properties. For instance, they are easier to study — they contain clearly defined cell types, with the cells organized into regular patterns, unlike the often disorganized tissues found in most current integrated models. And these alternative models are often built using bioengineering tools such as customized culture surfaces15 or tiny fluidic chambers through which chemicals can be channelled to control stem-cell growth and differentiation16. These tools allow researchers to tightly regulate the conditions that the models are grown in, making protocols more efficient, reproducible and scalable.
Non-integrated models, by definition, are not perfect replicas of normal development. But here we outline three situations in which non-integrated models could provide a good alternative to integrated ones.
Debate ethics of embryo models from stem cells
Proponents of making integrated human embryo models assert that only complete systems can tell us how the whole complex embryo grows. But many aspects of the second week of development can be studied without using integrated models. Researchers can efficiently grow models of the human epiblast using bioengineering tools15,16. Because these non-integrated approaches lack some extraembryonic cell types, they could never form a fetus with its placenta. Yet, they can still be used to model the development of the amniotic membrane (which is formed from epiblast cells), to analyse how the epiblast develops into different tissue layers during gastrulation, and to investigate the early stages of an embryo’s sperm and egg formation, which occurs during gastrulation.
Non-integrated models provide a straightforward way to study the early development of the placenta, which is limited or lacking in current integrated post-implantation models. Specifically, human trophoblast cells can be guided to form 3D structures, called trophoblast organoids, to study placenta development17.
The many challenges involved in growing integrated models past the 14-day mark can be avoided simply by using existing non-integrated models that mimic aspects of organ development. For instance, human stem cells can be coaxed to form somites — blocks of tissue from which the vertebrae develop (reviewed in refs 7,8). These models can be used to explore how the skeleton around the spinal cord forms and is altered in conditions such as scoliosis. Models of neuronal tissue developed from human stem cells are already providing insights into the mechanical forces involved in bending a sheet of cells into a closed neural tube, and into the origins of neural-tube defects18. And some non-integrated models can mimic formation of the spinal cord and of the gut tube, from which the respiratory and digestive systems form19,20.
Appropriately used, stem-cell-based models of human embryonic development could transform our understanding of how human life begins. We ask researchers to carefully consider whether there is a strong scientific rationale for replicating an entire human embryo from stem cells. Pushing ahead without careful deliberation risks a public backlash that could stall the progress in this exciting field.
[ad_2]
Source link