Is CRISPR safe? Genome editing gets its first FDA scrutiny
[ad_1]
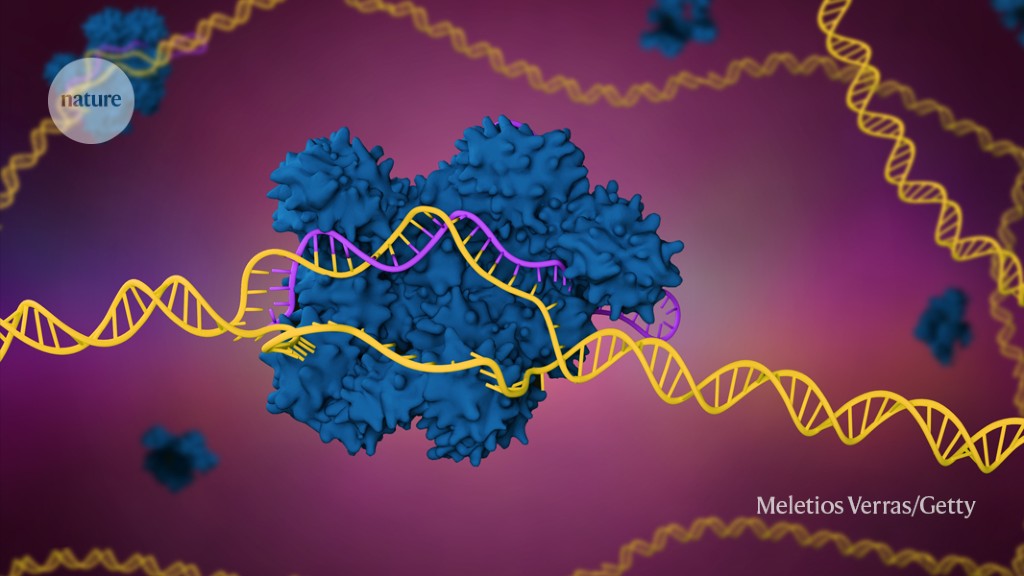
A sickle-cell disease therapy that harnesses the CRISPR-Cas9 genome editing system (artist’s illustration) is under review by US regulators.Credit: Meletios Verras/Getty
A therapy based on the CRISPR-Cas9 genome editing system could become the first of its kind to gain approval from the US Food and Drug Administration. But the treatment — designed to alleviate a painful blood condition — must first face intense scrutiny by the agency and its advisors.
On 31 October, external advisors to the US Food and Drug Administration (FDA) will meet to discuss a DNA-altering therapy for sickle-cell disease, a genetic condition that can cause misshapen blood cells and sometimes debilitating pain. The advisors’ discussions are likely to be laser-focused on safety data submitted by the treatment’s developers, Vertex Pharmaceuticals in Boston, Massachusetts, and CRISPR Therapeutics in Zug, Switzerland.
“The key to this is safety,” says Mark Walters, a paediatrician at the University of California, San Francisco, who has served on a steering committee advising the two companies on the clinical development of the treatment, called exagamglogene autotemcel (exa-cel), “That’s the question that could really affect decision making, and the safety information is still quite limited.”
Nature looks at what’s known and unknown about the safety of exa-cel and the other experimental genome editing therapies that are hot on its heels.
How exa-cel works
Sickle-cell disease is caused by abnormal forms of haemoglobin, the protein in red blood cells that transports oxygen. This altered haemoglobin makes blood cells misshapen and sticky, causing them to clump together and sometimes clog blood vessels. Clogged vessels deprive tissues of oxygen, potentially causing long-lasting damage and episodes of searing pain that are called vaso-occlusive crises.
Quest to use CRISPR against disease gains ground
Exa-cel aims to stop these by switching on another form of haemoglobin normally made only in developing fetuses. Production of this fetal haemoglobin is typically shut off soon after birth by a gene called BCL11A. Exa-cel disables BCL11A, allowing fetal haemoglobin production to resume. This provides some haemoglobin that is not misshapen, and mutes the effects of the abnormal form.
Vertex and CRISPR Therapeutics reported that 9 months after treatment, 39 of the 40 participants in their clinical trial had not had a single vaso-occlusive crisis. Before their treatment, they’d had an average of about four each year.
To apply exa-cel, clinicians first collect blood-producing stem cells from a person with sickle-cell disease. The cells are then treated with the genome editor, which includes the Cas9 enzyme for cutting DNA and a molecule that guides the enzyme to the target stretch of DNA within the BCL11A gene.
Once at that region, the Cas9 enzyme cuts both strands of DNA. The cell’s natural DNA-repair mechanisms then stitch the strands back together again. But those mechanisms are prone to making mistakes, which means that they often introduce errors in the DNA sequence. These errors can disable BCL11A and release the brakes on fetal haemoglobin production.
Edits that miss the mark
But Cas9 sometimes slices DNA at other regions in the genome that are similar to its target. The cell’s repairs to that ‘off-target’ DNA can introduce unwanted mutations. That scenario is an important consideration for CRISPR-Cas9 therapies, because a mutation in a critical gene could cause cancer or other disorders, even though the risk might be small, says Samira Kiani, a synthetic biologist at the University of Pittsburgh School of Medicine in Pennsylvania.
Briefing documents released 27 October suggest that FDA examiners have zeroed in on this possibility, and it is slated to become a key discussion point when advisors meet to discuss the risks and benefits of exa-cel next week.
In particular, the FDA flagged two potential shortcomings in assays that Vertex and CRISPR Therapeutics used to determine exa-cel’s risk of causing off-target changes. In one assay, researchers searched a database of genomes to find regions that are similar to exa-cel’s CRISPR-Cas9 target site and that therefore might be mistakenly cleaved by the Cas9 enzyme. The scientists then gauged the risk of changes at these sites.
CRISPR gene editing in human embryos wreaks chromosomal mayhem
Most of the sites did not raise concerns, says Walters. But sickle-cell disease predominantly affects people of African descent, and FDA examiners are concerned that the genetic diversity in these populations was not captured in the genomes the companies searched. That means that there could be genetic sequences in those populations that might be targeted by Cas9 but were missed by the analysis.
And exa-cel has only been tested in 40 people — too few to assess the risk of off-target changes, says Akshay Sharma, a bone marrow transplant specialist at St. Jude Children’s Research Hospital in Memphis, Tennessee. “We’re going to deal with so much more genetic diversity which we haven’t accounted for in these trials,” Sharma says.
The FDA’s examiners also worried that the companies’ assays in live cells for off-target effects did not include enough cells sampled from people with sickle-cell disease.
Cancer concern
For Walters, there is a bigger concern than off-target edits: two participants in a clinical trial of a separate gene-altering therapy for sickle-cell disease later developed blood cancers. In that trial, conducted by Bluebird Bio in Cambridge, Massachusetts, a virus shuttled copies of a non-sickled form of haemoglobin into blood stem cells.
Subsequent investigations showed that the cancers were not caused by the virus. That has fuelled speculation that similar cases could crop up among those who receive other therapies, such as exa-cel, that involve modifying blood stem cells from people with sickle-cell disease and then reinfusing them.
Gene therapy is facing its biggest challenge yet
Some studies have shown that people with sickle-cell disease might be more prone to malignant blood cancers. If precancerous cells were among those that were removed, altered and reinfused, the treatment might have somehow given them a boost that allows them to take over the population and become cancerous. “This is a principal, long-term, potential complication that we’re going to have to sort out,” says Walters.
Vertex and CRISPR Therapeutics have proposed to follow trial participants for 15 years after their treatment, to look for long-term impacts of their treatment. In the meantime, it is important that physicians inform their patients of the risks, says Tosin Ola, founder of the advocacy group Sickle Cell Warriors in San Marcos, California. “These are things that I know because I’m a nurse and I do advocacy,” she says. “But a typical mom who is worried for her kid who has sickle cell, she won’t know it. She’ll just sign up.”
Sharma shares those concerns but, like Olma, acknowledges that the development of treatments like exa-cel should continue, despite the unknowns. “There are patients right now who are still suffering, and they need some sort of therapy,” says Sharma. “We’ve got to find something for them.”
[ad_2]
Source link