CAR-T therapy forces autoimmune diseases into remission
[ad_1]
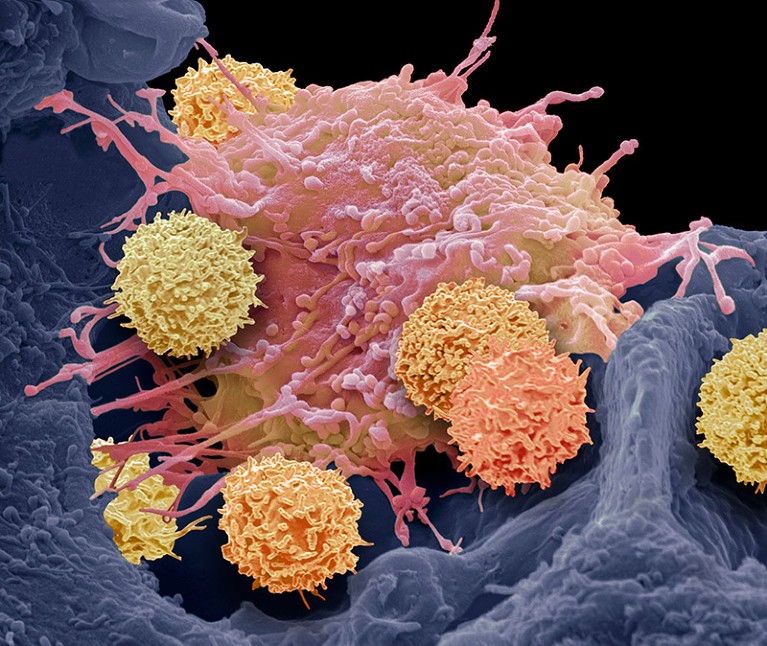
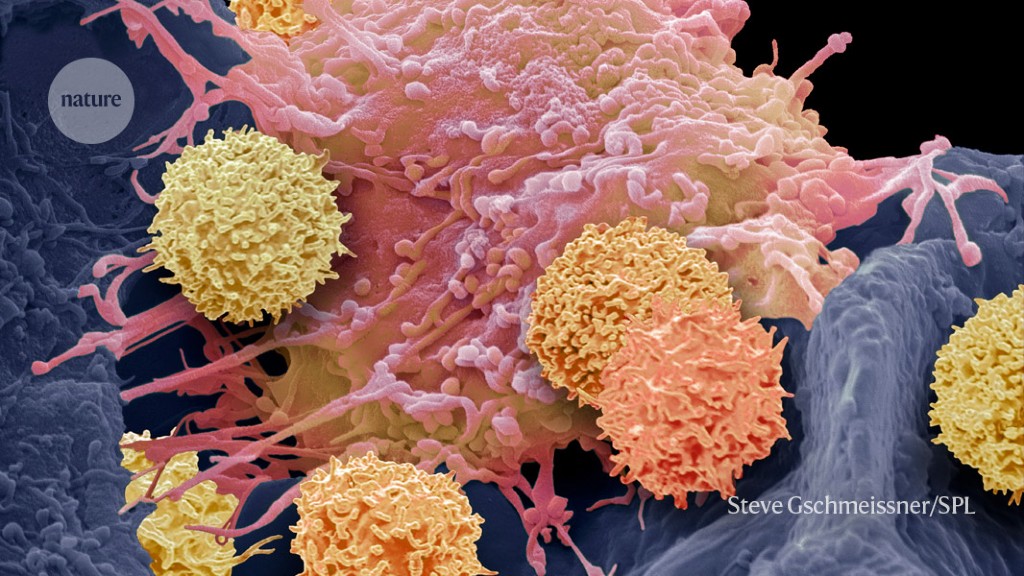
T cells (smaller cells) can be engineered to recognize cancer cells — and also other immune cells.Credit: Steve Gschmeissner/Science Photo Library
Engineered immune cells have given 15 people with once-debilitating autoimmune disorders a new lease on life, free from fresh symptoms or treatments. The results raise hopes that the approach — called CAR-T-cell therapy — might one day be extended to a variety of other conditions fuelled by rogue immune cells that produce antibodies against the body’s own tissues.
All 15 participants, who each had one of three autoimmune conditions, have remained disease-free or nearly so since their treatment, according to data presented on 9 December at the American Society of Hematology meeting in San Diego, California. The first participants were treated more than two years ago.
These successes, although preliminary, have been electric, says Marco Ruella, an oncologist at the University of Pennsylvania in Philadelphia. “We’re all excited,” he says. “There’s a lot of potential.”
Bespoke immune cells
CAR-T therapies harness the immune players called T cells. T cells are removed from the person being treated, genetically engineered to produce proteins called chimeric antigen receptors (CARs) and then reintroduced to the person’s body. In many therapies, the T cells are tailored to recognize a protein made by immune cells called B cells. When reintroduced, the CAR T cells will target the B cells for destruction — a useful feature for treating cancers caused by abnormal B cells.
The race to supercharge cancer-fighting T cells
B cells also drive some autoimmune disorders by making antibodies that attack healthy tissue. In 2019, researchers showed that CAR T cells that recognize B cells reduced symptoms in mice with a disease similar to lupus, an autoimmune disorder that affects a variety of organs1.
Around the same time, researchers at University Hospital Erlangen in Germany were setting up their own CAR-T centre to provide cancer treatment. During a meeting at the centre, a rheumatologist asked the cancer specialists for advice about a young woman with a form of lupus called systemic lupus erythematosus. Several of her organs were failing; her doctors estimated that she did not have long to live. The young woman insisted that they try something new.
High-risk approach
The team thought of the mouse study but baulked at trying it in people. CAR-T therapy can have severe side effects, and recipients must first undergo intensive chemotherapy that kills off many of their existing immune cells. “At the beginning we were quite scared,” said team member Fabian Müller, an oncologist at the Friedrich–Alexander University of Erlangen–Nuremberg, at a press conference before he presented the work at the San Diego meeting. The woman was adamant that they try.
That first participant — and the others who followed — experienced relatively minor adverse effects, Müller reported at the conference. The Erlangen team eventually used the method to treat two other autoimmune disorders: systemic sclerosis and idiopathic inflammatory myositis. The successes continued.
Cancer treatments boosted by immune-cell hacking
Other groups have since taken up the approach and reported similar results. Earlier this month, another team added a fourth autoimmune disorder called myasthenia gravis to the list of successes2. Researchers are beginning to wonder how long the final list will be. “We’re just at the beginning,” says Marcela Maus, who designs CAR-T therapies against cancer at Massachusetts General Hospital in Boston. “There is so much that can be done that was unthinkable just a decade ago.”
At this stage, however, it’s unclear how much of this success is due to the CAR-T therapy as opposed to the chemotherapy that killed many of the participants’ pre-existing immune cells, cautions Ruella. That might have helped to wipe out the errant B cells.
For now, Müller lapses into a dreamy smile as he marvels over the remarkable recoveries he has seen: the man who struggled to walk 10 metres before his treatment and now routinely walks 10 kilometres around town, for example. “These are young people that have been spending more time with their doctors than with their friends,” he says. “They would describe their breakfast as a handful of pills that they are just shoving in.”
“And it’s all gone,” he says. “From the physician perspective, it’s pretty much the most pleasing thing.”
[ad_2]
Source link